The European Investment Bank (EIB) will provide €20 million in venture debt financing to German medical technology startup Protembis, which will use the funds to further develop a new system for protecting the brain during certain cardiac procedures.
Specifically, the funding is intended to enable clinical trials, research, development and market launch of the ProtEmbo embolic protection system. ProtEmbo is a filtering device that prevents embolic particles released during left heart surgery, transcatheter aortic valve implantation (TAVI), from entering the arteries supplying blood to the brain, with the goal of preventing risks such as stroke and cognitive decline.
EIB Vice-President Nicola Beer said: “This agreement underlines our commitment to companies like Protenvis, which are committed to the health and well-being of Europeans. With innovative technology developed in Europe, Protenvis will help protect patients from serious complications such as cerebral embolism.”
The EIB financing is supported by the InvestEU programme, which aims to stimulate more than €372 billion in additional investment in new technologies across Europe by 2027. The agreement supports InvestEU’s goal of promoting research, development and innovation.
Protenvis Managing Directors Karl von Mangoldt and Konrad Rasmus said: “We are pleased to announce the signing of the agreement with the EIB. We would particularly like to highlight the efforts and high level of competence and professionalism of the EIB team. At the same time, we would like to thank our existing investors and the Advisory Board for their strong support of this additional financing.”
TAVI is an x-ray guided procedure to replace a narrowed aortic valve that has stopped opening completely. The procedure is minimally invasive, so the access points are smaller than in open-heart surgery. By 2025, it is expected that approximately 430,000 patients with severe heart valve defects will undergo this procedure worldwide.
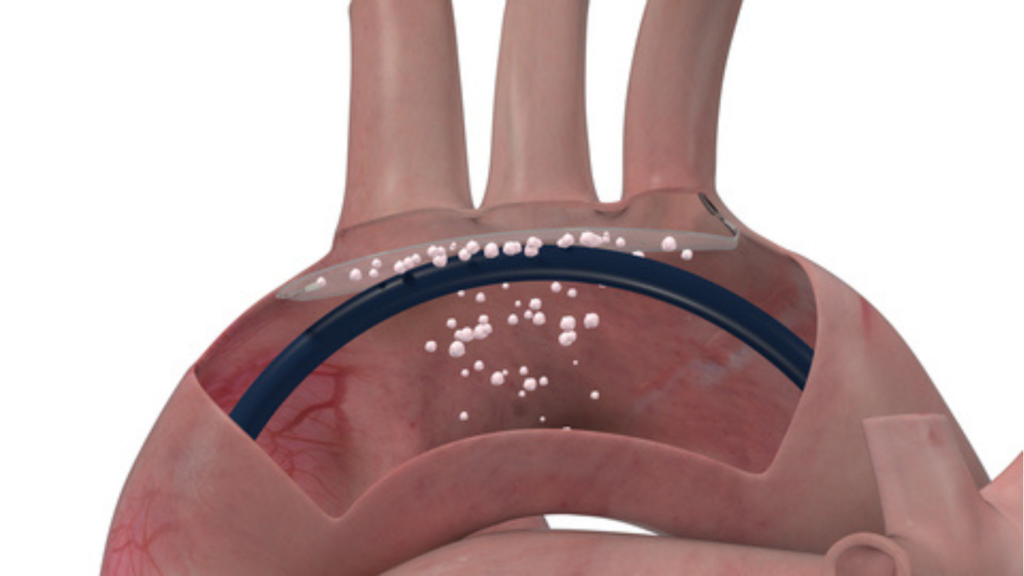
The big risk is that particles deposited in the vessel walls of the aortic arch and the old aortic valve can detach. These particles can enter the brain through the three large arteries that branch off from the upper vessel walls of the aortic arch, where they can block narrow cerebral blood vessels and damage tissue, causing strokes and loss of cognitive ability.
ProtEmbo is inserted through an artery in the left wrist for TAVI. The filter lines the upper vessel wall of the aortic arch, protecting the brain from dissolved particles.
In March 2024, Protembis closed a €30 million Series B funding round to advance its approval trial, the PROTEMBO trial, following investigational product exemption (IDE) approval by the US FDA. The trial is expected to enroll 250-500 patients undergoing TAVI in Europe or the US. The randomized trial aims to demonstrate the superiority of the ProtEmbo system over a hybrid control arm, half of which will receive no embolic prophylaxis and the other half will receive the comparable approved product, Sentinel.
ProtEmbo and Protembis: The ProtEmbo cerebral protection system is an intra-aortic filter that provides full brain protection from embolic particles that may be released during transcatheter aortic valve implantation (TAVI). It is a low-profile, non-thrombogenic system that protects the cerebral vessels. To ensure optimal placement and stability, it is inserted via the left radial artery, meaning it does not conflict with TAVI systems that are typically inserted via the femoral artery.
The ProtEmbo ® brain protection system has been developed by Protembis GmbH. The medical technology start-up wants to offer a simple and reliable solution to prevent brain injuries during left ventricular surgery in order to improve patients’ quality of life. This will also reduce the medical costs associated with brain injuries during such procedures. ProtEmbo ® is currently in clinical trials.
sauce