Selection of a novel Lp-PLA2 inhibitor, DPT0415, for the treatment of DR and DME. DPT0415 is a highly potent, selective and safe Lp-PLA2 inhibitor that exhibits sufficient target binding at a dose of 0.3 mpk and exerts robust efficacy in STZ-induced rat DR model.
The project will further validate the capabilities of DP Technology’s drug design platform RiDYMO.
BEIJING, July 8, 2024 /PRNewswire/ — DP Technology, an “AI for Science” paradigm-driven company, today announced that it has named DPT0415, a novel small molecule targeting lipoprotein-associated phospholipase A2 (Lp-PLA2), as a preclinical candidate for the treatment of diabetic retinopathy (DR) and diabetic macular edema (DME).
Lp-PLA2 belongs to group VII of the PLA2 superfamily and is secreted primarily by macrophages and circulates in the blood as complexes with low-density lipoproteins (LDL) and high-density lipoproteins (HDL).[1]Lp-PLA2 primarily hydrolyzes oxidized phospholipids on low-density lipoprotein (LDL) to generate lysophosphatidylcholine (lyso-PC), which induces vascular inflammation and leads to damage of the blood-retinal barrier (BRB).[2].
DR is a microvascular complication of diabetes and is the leading cause of vision loss in middle-aged and older people. One-third of people with diabetes have DR.[3]DME can occur at any stage of DR and is caused by the accumulation of excess fluid and lipids in the macula due to breakdown of the blood-retinal barrier. If the swelling spreads to the fovea, patients will present with metamorphopsia and vision loss.[4].
The advent of intraocular anti-vascular endothelial growth factor (anti-VEGF) drugs has revolutionized the treatment of DME, but many patients with DME never fully clear fluid despite multiple injections.[5]This is probably because DR is also an inflammatory disease, and many cytokines and chemokines are involved in the process.[6]Therefore, molecular mechanisms other than VEGF need to be investigated. Oral administration of darapladib, an Lp-PLA2 inhibitor, slightly reduced edema and improved visual acuity in a phase IIa study of DME.[7].
DPT0415 is a highly potent, selective and safe Lp-PLA2 inhibitor with a novel scaffold. It showed sufficient target binding at 0.3 mpk and reestablished retinopathy in STZ-induced rat DR model. The compound showed higher potency than darapladib, better ADME and physicochemical properties, and provided a sufficient safety margin in preclinical studies.
“The current standard of care for DME requires intraocular injections of VEGF antibodies to preserve vision. Due to the invasive route of administration, patients tend to delay treatment until later in the disease,” said Xiaomin Zhang, head of drug discovery at DP Technology. “As an oral therapy for the treatment of DR and DME, DPT0415 may address inflammation and vascular leakage by targeting Lp-PLA2, improving vision. Furthermore, the Lp-PLA2 inhibitor DPT0415 has the potential to significantly change the treatment paradigm by treating DR earlier to achieve better outcomes.”
“DP Technology is a pioneer in the AI for Science paradigm, focusing on integrating ‘AI + Simulation + Experiment’ to address unmet medical needs,” said Weijie Sun, founder and CEO of DP Technology. “The successful discovery of DPT0415 was enabled by multiple modules such as Uni-FEP and Uni-QSAR in our RiDYMO® platform to optimize potency and ADME properties. The practical application of these algorithms represents an important step towards realizing our AI for Science vision. We look forward to working with our experienced partners to advance this program to the next milestone.”
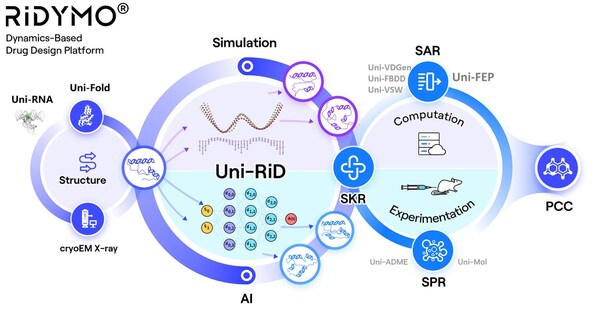
The RiDYMO® drug design platform integrates various AI and physics algorithms, specializing in developing drugs for “undruggable” targets and “best-in-class” molecules. One of its core algorithms, Reinforced Dynamics (RiD), has a significant advantage in the sampling efficiency of molecular dynamics simulations. By taking full advantage of the high-dimensional representation capabilities of neural networks, RiD can efficiently capture the dynamic structural changes of complex biomolecular systems.
The RiDYMO® platform aims to study the dynamics of biological systems and reveal hidden binding sites, covering a wide range of challenging systems, including protein-protein interactions (PPIs), intrinsically disordered proteins (IDPs), membrane proteins, RNA, etc. Its validity has been confirmed through validation against challenging targets, including c-Myc protein, c-Myc RNA, GPX4 protein, and Kv1.3 protein.
About DP Technology
DP Technology is a new paradigm-driven company called “AI for Science” that is dedicated to applying artificial intelligence and molecular simulation algorithms to solve important scientific problems by combining advanced computational methods.
Leveraging DP Technology’s dynamics-based drug design platform RiDYMO®, the company has built a world-leading hit discovery platform. The team is focused on three areas: CNS, oncology and autoimmune diseases, establishing external collaborations and building a strong internal pipeline.
For collaboration or further information, please contact Dr. Xiaomin Zhang at zhangxm@dp.tech.
References:
1. Current Medicine. Des. 20, 6256-6269 (2014).
2. Atherosclerosis. Thrombosis. Blood vessels. Biology. 25, 923-931 (2005).
3. Diabetes Care 35, 556-564 (2012).
4. Ophthalmology 122, 1375-1394 (2015).
5. Ophthalmology 117, 1064-1077 (2010).
6. Seminars in Immunopathology 30, 65-84 (2008).
7. Ophthalmology 122, 990-996 (2015).